Biovanta Double Action
Dosage form: spray
Ingredients: ASPIRIN 6mg in 0.1mL
Labeler: Applied Biological Laboratories Inc.
NDC code: 73678-160
Medically reviewed by Drugs.com. Last updated on Aug 23, 2023.
Acetyl Salicylic Acid (NSAID) 6mg
- nonsteroidal anti-inflammatory drug
Analgesic
For the temporary relief of minor aches and pains associated with a sore throat.
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye’s syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if the user:
- has had stomach ulcers or bleeding problems
- takes a blood thinning (anticoagulant) or steroid drug
- takes other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- takes more or for a longer time than directed
- is age 60 or older
- has 3 or more alcoholic drinks every day while using this product
If pregnant or breast-feeding, always ask your healthcare provider before use.
Do not use:
- if you have stomach problems (such as heartburn, upset stomach, or stomach pain) that persist or recur, or if you have ulcers or bleeding problems, unless directed by a doctor
- If allergic to milk and/or egg white, or have ever had an allergic reaction to this product or any of its ingredients.
A persistent cough may be a sign of a serious condition. If cough persists for more than 1 week, tends to recur, or is accompanied by fever, rash, or persistent headache, consult your healthcare provider.
Children Warning: Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
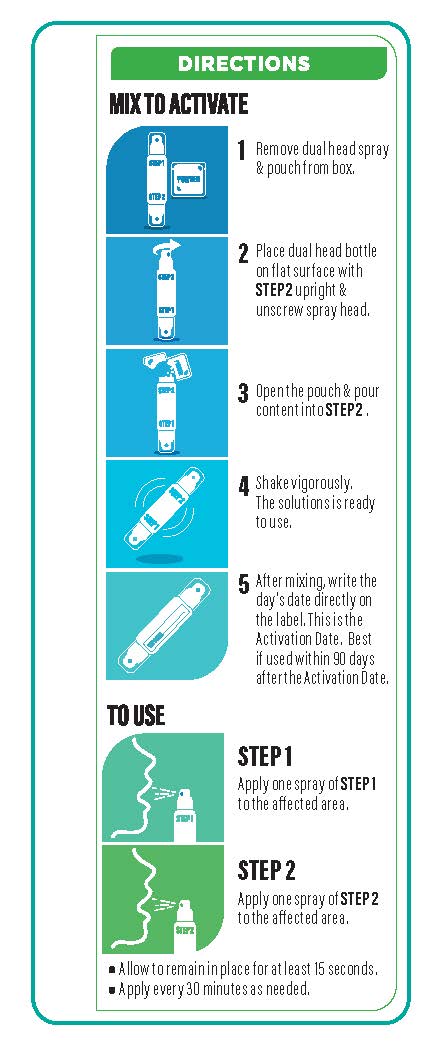
- Apply one spray of Step1 and one spray of Step2 to the affected area every thirty minutes or as needed.
- Allow to remain in place for at least 15 seconds.
- Once mixed, do not use after 90 days.
■ Store at room temperature.
■ Check unmixed product expiration date. Once mixed, fill in activation date on spray label watermark area. Do not use after 90 days after activation.
■
Tamper Evident: Do not use if imprinted shrink band is broken or missing.
Aloe, Ethanol, Glycerin, Lactoferrin, Lysozyme, Menthol, Propylene Glycol, Sodium Chloride, Sorbic Acid, Water
| BIOVANTA DOUBLE ACTION
acetylsalicylic acid spray |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Applied Biological Laboratories Inc. (080512733) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.