Epuris Prescribing Information
Package insert / product label
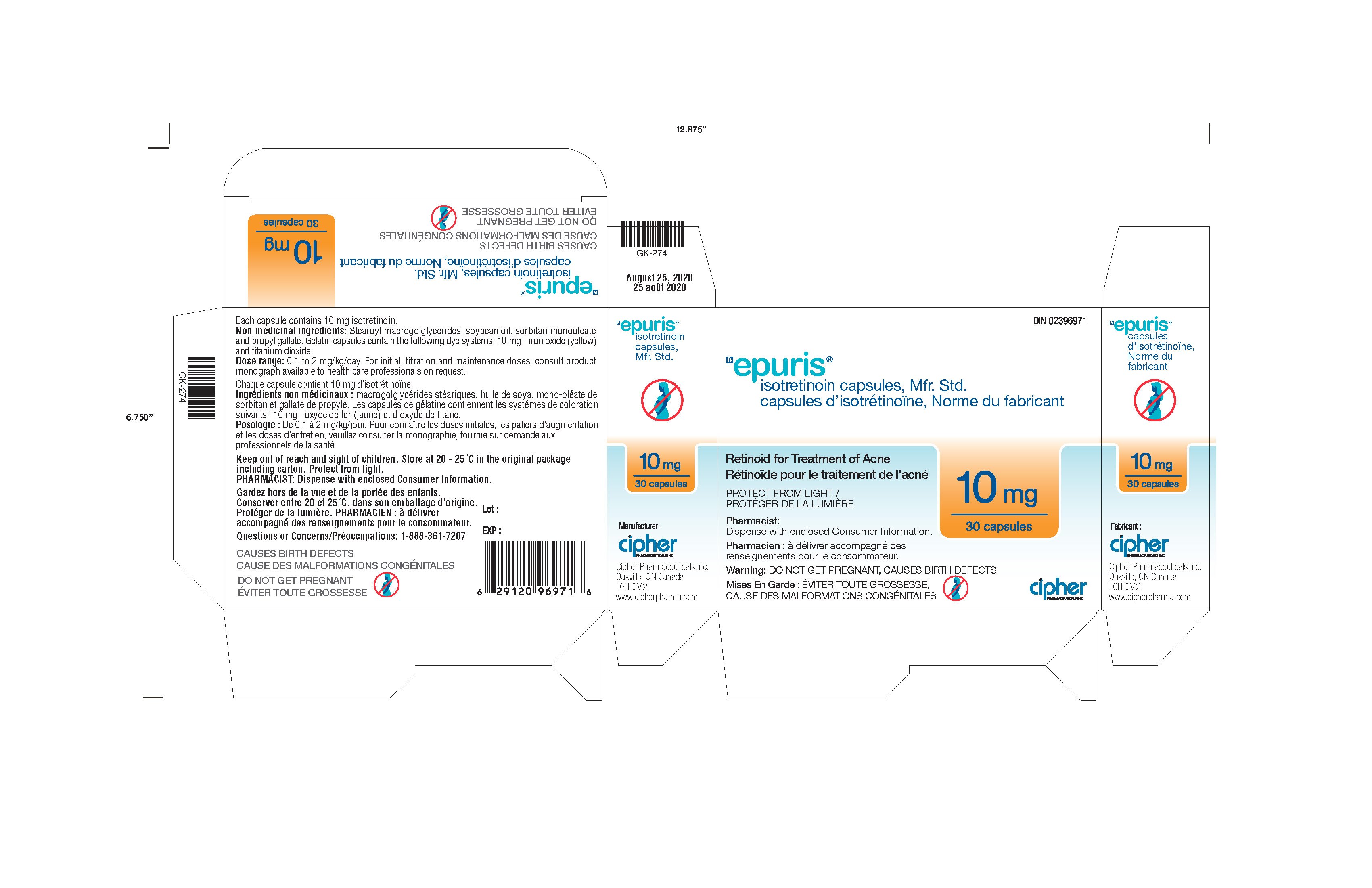
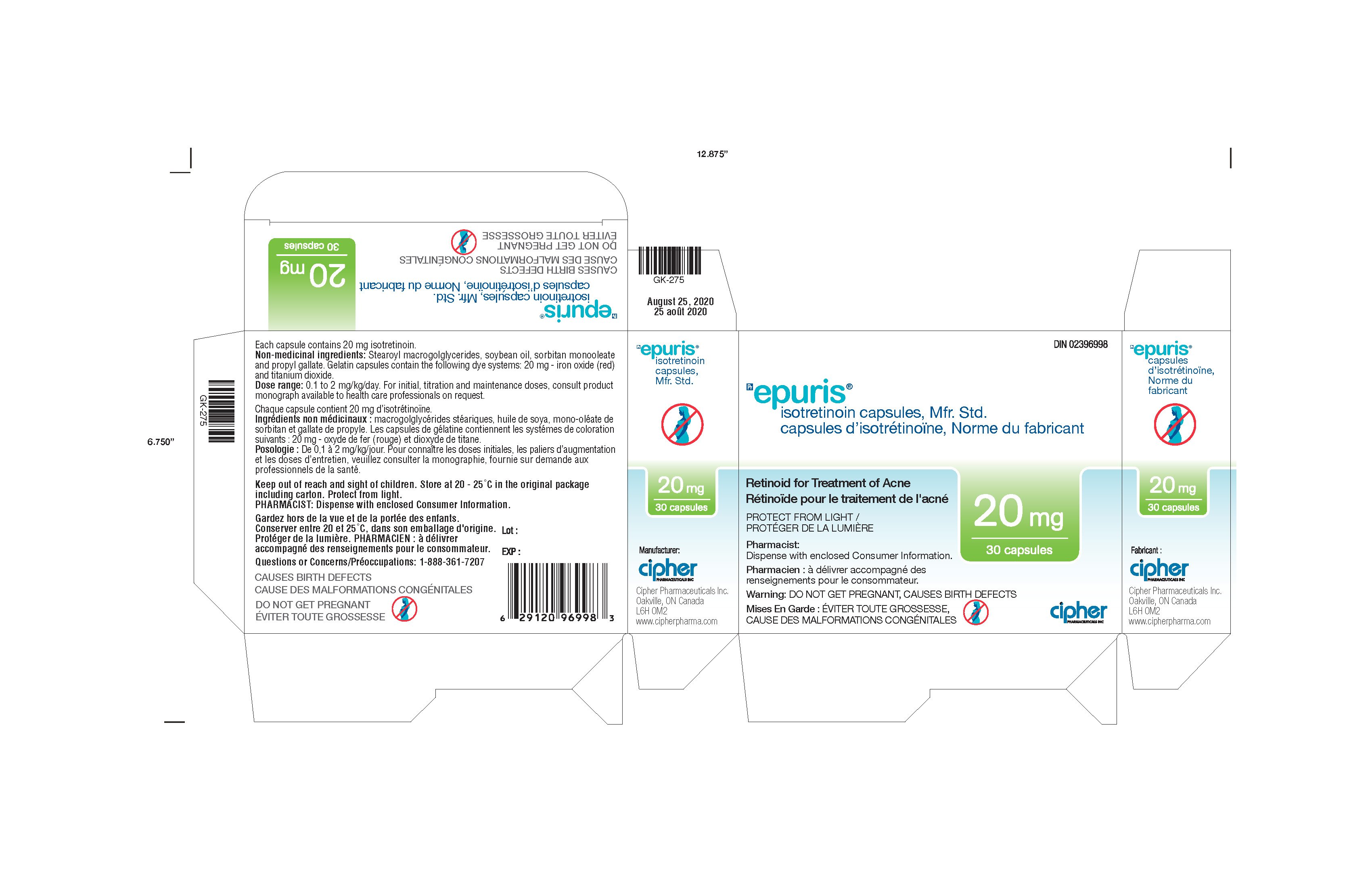
Generic name: isotretinoin
Dosage form: capsule
Drug classes: Miscellaneous antineoplastics, Miscellaneous uncategorized agents
Medically reviewed by Drugs.com. Last updated on Jan 23, 2024.
| EPURIS
isotretinoin capsule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| EPURIS
isotretinoin capsule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| EPURIS
isotretinoin capsule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| EPURIS
isotretinoin capsule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Galephar Pharmaceutical Research Inc. (003551624) |
| Registrant - Galephar Pharmaceutical Research Inc. (968996160) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Galephar Pharmaceutical Research Inc | 968996160 | analysis(66277-337, 66277-338, 66277-339, 66277-340) , manufacture(66277-337, 66277-338, 66277-339, 66277-340) , pack(66277-337, 66277-338, 66277-339, 66277-340) | |
Related/similar drugs
doxycycline, clindamycin topical, erythromycin topical, tetracycline, Tazorac
Frequently asked questions
- What are the most common skin conditions? (with photos)
- Can Accutane cause permanent liver damage?
- Is Absorica the same as Accutane?
- How long does Absorica take to work?
- Isotretinoin - Does accutane help to remove scars from old pimples?
More about isotretinoin
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (1,053)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Support group
- Drug class: miscellaneous antineoplastics
- Breastfeeding
Patient resources
Professional resources
Other brands
Accutane, Claravis, Zenatane, Absorica, ... +2 more