Claro (Canada)
This treatment applies to the following species: Company: Elanco
Company: Elanco
Florfenicol, Terbinafine hydrochloride, and Mometasone furoate Otic Solution
VETERINARY USE ONLY
For Use In Dogs Only
DIN 02472953
Description
CLARO contains 16.6 mg/mL florfenicol, 14.8 mg/mL terbinafine (equivalent to 16.6 mg/mL terbinafine hydrochloride) and 2.2 mg/mL mometasone furoate.
THERAPEUTIC CLASSIFICATION:
Antibiotic, antifungal and anti-inflammatory.
Claro Indications
CLARO is indicated for the treatment of otitis externa in dogs associated with susceptible strains of yeast (Malassezia pachydermatis) and bacteria (Staphylococcus pseudintermedius).
Dosage and Administration
Shake before use.
CLARO should be administered by veterinary personnel in clinic. Wear eye protection when administering CLARO (See CAUTIONS and WARNINGS). Splatter may occur if the dog shakes its head following administration. Persons near the dog during administration should also take steps to avoid ocular exposure. Verify the tympanic membrane is intact prior to administration.
Administer one dose (1 dropperette) per affected ear. The duration of effect should last 30 days.
1. Clean and dry the external ear canal before administering the product.
2. Verify the tympanic membrane is intact prior to administration.
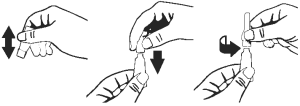
3. Remove single dose dropperette from the package.
4. While holding the dropperette in an upright position, remove the cap from the dropperette.
5. Turn the cap over and push the other end of the cap onto the tip of the dropperette.
6. Twist the cap to break the seal and then remove cap from the dropperette.
7. Screw the applicator nozzle onto the dropperette.
8. Insert the tapered tip of the dropperette into the affected external ear canal and squeeze to instill the entire contents (1 mL) into the affected ear.
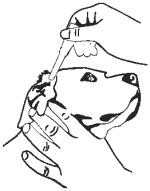
9. Gently massage the base of the ear to allow distribution of the solution. Restrain dog to minimize post application head shaking to reduce the potential for splatter of product and accidental eye exposure in people and dogs.
10. Repeat with other ear as prescribed.
Cleaning the ear after dosing may affect product effectiveness.
Appropriate diagnosis and control of underlying causes of otitis externa should be addressed.
Whenever possible, the use of CLARO should be based on identification of the infectious agent(s).
Contraindications
Do not use in dogs with known tympanic membrane perforation (see CAUTIONS).
Do not administer orally.
Do not use in dogs with known or suspected hypersensitivity to florfenicol, terbinafine hydrochloride, mometasone furoate, other corticosteroids or any excipients.
CAUTIONS:
Avoid accidental contact with the dog’s eyes during administration of CLARO by restraining the dog to minimize head shaking. In case of exposure to the eye, rinse with abundant quantities of water. If any eye irritation is seen after treatment, seek veterinary care.
The integrity of the tympanic membrane should be confirmed before administering the product. In some cases, CLARO has been associated with the rupture of the tympanic membrane. Reevaluate the dog if hearing loss or signs of vestibular dysfunction are observed during treatment. Signs of internal ear disease such as head tilt, vestibular signs, ataxia, nystagmus, facial paralysis, and keratoconjunctivitis sicca have been reported with the use of CLARO.
Use of topical otic corticosteroids has been associated with adrenocortical suppression and iatrogenic hyperadrenocorticism in dogs (see ANIMAL SAFETY).
The safe use of CLARO in dogs used for breeding purposes, during pregnancy or lactation has not been evaluated.
CLARO should be used with caution in dogs with impaired hepatic function (see ANIMAL SAFETY).
Warnings
Keep out of reach of children. Not for use in humans. In case of accidental ingestion, contact a physician immediately. When handling this product, special caution should be taken to avoid oral exposure and direct contact with eyes or skin. CLARO may cause eye injury and irritation. Wear eye protection when administering CLARO and restrain the dog to minimize post application head shaking. In case of accidental contact with eyes, rinse with abundant quantities of water and seek medical attention. In case of accidental skin contact, wash area thoroughly with water. Humans with known hypersensitivity to florfenicol, terbinafine hydrochloride, or mometasone furoate should not handle this product.
Adverse Reactions
Although not all adverse reactions are reported, the following information is based on voluntary post-approval drug experience reporting. It is generally recognized that this results in significant under-reporting. The adverse events listed here reflect reporting and not necessarily causality.
The adverse events reported in CLARO in dogs are presented below in decreasing order of reporting frequency. By system, these clinical signs were reported for CLARO rarely (1-10 reports per 10,000 doses sold) or very rarely (< 1 report per 10,000 doses sold) and include:
Systemic disorders: lack of efficacy*, lethargy, anorexia
Ear and labyrinth disorders: internal ear disorder NOS (head tilt, vestibular disorders), deafness, ear discharge, ear canal erythema, ear canal inflammation, tympanic rupture
Neurological disorders: ataxia, nystagmus, cranial nerve disorder (facial paralysis)
Digestive tract disorders: vomiting
Eye disorders: keratoconjunctivitis sicca (KCS), corneal ulcer, blepharospasm
Behavioural disorders: head shaking
*Efficacy of the product may be affected by confounding factors present at the time of product application, such as unaddressed underlying causes or predisposing and perpetuating factors of otitis externa. This product should be used according to the label and based on identification of the infectious agent(s) (see DOSAGE AND ADMINISTRATION).
CLARO is not approved for use in cats. The adverse events reported following extra-label use in cats are presented below in decreasing order of frequency:
Ataxia, anorexia, internal ear disorder (head tilt and vestibular), Horner’s syndrome (third eyelid prolapse and miosis), nystagmus, lethargy, anisocoria, head shake, emesis, tympanic rupture and deafness.
To report suspected adverse drug events or for technical assistance, contact Elanco Canada Limited at 1-800-265-5475.
Information For Dog Owners:
Owners should be aware that adverse reactions may occur following administration of CLARO and should be instructed to observe the dog for signs such as ear pain and irritation, vomiting, head shaking, head tilt, incoordination, eye pain and ocular discharge (see ADVERSE REACTIONS). Owners should be advised to contact their veterinarian if any of the above signs are observed.
Owners should also be informed that splatter may occur if the dog shakes its head following administration of CLARO, which may lead to ocular exposure. As a result, eye injuries in humans and dogs have been reported including corneal ulcers. Owners should be careful to avoid ocular exposure (see CAUTIONS and WARNINGS).
Clinical Pharmacology
CLARO Otic Solution is a fixed combination of three active substances: florfenicol (antibiotic), terbinafine (antifungal) and mometasone furoate (steroidal anti-inflammatory). Florfenicol is a bacteriostatic antibiotic which acts by inhibiting protein synthesis. Terbinafine is an antifungal which selectively inhibits the early synthesis of ergosterol. Mometasone furoate is a glucocorticosteroid with anti-inflammatory activity.
MICROBIOLOGY:
The compatibility and additive effect of each of the components in CLARO solution was demonstrated in a component effectiveness and non-interference study in vitro study of organisms collected from clinical cases of otitis externa in dogs enrolled in the clinical effectiveness study determined that florfenicol and terbinafine hydrochloride inhibit the growth of bacteria and yeast commonly associated with otitis externa in dogs. No consistent synergistic or antagonistic effect of the two antimicrobials was demonstrated. The addition of mometasone furoate to the combination did not impair antimicrobial activity to any clinically significant extent.
In a field study (see EFFECTIVENESS), at least 10 isolates from successfully treated cases were obtained for S. pseudintermedius and M. pachydermatis.
SAFETY AND EFFICACY INFORMATION:
Effectiveness
In a well-controlled, double-masked field study, CLARO was evaluated against a vehicle control in 221 dogs with otitis externa. One hundred and forty six dogs were treated with CLARO and 75 dogs were treated with the vehicle control. All dogs were evaluated for safety. Treatment (1 mL) was administered once on Day 0 to the affected ear(s). Prior to treatment, the ear(s) was cleaned with saline. The dogs were evaluated on Days 0, 7, 14, and 30. Blood work and urinalysis were obtained on Day 0 pre-treatment and Day 30 at study completion. Four clinical signs associated with otitis externa were evaluated: erythema, exudate, swelling, and ulceration. Success was based on clinical improvement at Day 30. Of the 183 dogs included in the effectiveness evaluation, 72.5% of dogs administered CLARO solution were successfully treated, compared to 11.1% of the dogs in the vehicle-control group (p=0.0001).
Safety
In a target animal safety study, CLARO was administered aurally to 12-week-old Beagle puppies (4 dogs/sex/group) at 0X, 1X, 3X, and 5X the recommended dose once every 2 weeks for a total dosing period of 28 days (3 times the treatment duration). No clinically relevant treatment-related findings were noted in hearing tests, body weight, weight gain, or food consumption. CLARO administration was associated with post-treatment ear wetness or clear aural exudate, increased absolute neutrophil count, decreased absolute lymphocyte and eosinophil counts, suppression of the adrenal cortical response to adrenocorticotropic hormone (ACTH) stimulation, decreased adrenal weight and atrophy of the adrenal cortex, increased liver weight with hepatocellular enlargement/cytoplasmic change, and decreased thymus weight. Other potentially treatment-related effects included mild changes to aspartate aminotransferase, total protein, inorganic phosphorus, creatinine, and calcium.
Storage
Store at 15°C-25°C. Excursions permitted to 30°C.
How Supplied
CLARO is supplied in a single-use dropperette in a blister. Each dropperette contains one 1 mL dose.
CLARO is available in cartons of 2 or 10 dropperettes.
MANUFACTURED FOR:
Elanco Canada Limited, 1919 Minnesota Court, Suite 401, Mississauga, Ontario L5N 0C9
DATE: January 2024
CLARO, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
© 2024 Elanco or its affiliates.
CONTAINS ANTIMICROBIAL
USE RESPONSIBLY
24Jan2024
CPN: 1231208.4
1919 MINNESOTA COURT, SUITE 401, MISSISSAUGA, ON, L5N 0C9
| Customer Service: | 800-265-5475 | |
| Fax: | 519-821-7831 | |
| Website: | www.elanco.ca | |
| Email: | elancocanadacustomerservice@elancoah.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
