GuaifenJect
This treatment applies to the following species: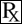 Company: Henry Schein® Animal Health
Company: Henry Schein® Animal Health
50 mg per mL
ANADA 200-230, Approved by FDA
For Intravenous Use in Horses Only
GuaifenJect Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
Guaifenesin, 3 - (o-Methoxyphenoxy) 1,2-propanediol, is a white to slightly gray, crystalline powder having a bitter taste. It may have a slight characteristic odor. Each mL of the injection contains: 50 mg guaifenesin, 50 mg dextrose (anhydrous), 20 mg propylene glycol, 50 mg dimethylacetamide (parenteral grade), 0.75 mg edetate disodium, water for injection q.s.
ACTIONS Guaifenesin is a guaiacol derivative closely related to mephenesin. The propanediol derivatives are central-acting skeletal muscle relaxants which selectively depress transmission of nerve impulses at the internuncial neurons of the spinal cord, brainstem, and subcortical regions of the brain.
Propanediol derivatives have a wide margin of safety, the toxic dose is three times greater than the recommended dose. Respiration is not severely depressed, and the function of the diaphragm is uninterrupted (1) (2). Tidal volume is slightly decreased and minute volume remains normal. In addition to skeletal muscle, the pharyngeal and laryngeal muscles are sufficiently relaxed for easier surgical intubation procedures. At the beginning of relaxation, there is a slight fall in blood pressure, however, throughout the relaxation period the heart function is unchanged. There is a slight increase in gastrointestinal activity. There is no apparent impairment of lung and kidney functions.
With the recommended administration and dosage of GuaifenJect, induction is usually smooth and rapid (two to four minutes) providing a duration of action between 15 to 25 minutes. A slightly transient hemolysis occurs at a 5% concentration (3) (4) Higher concentrations cause intravascular hemolysis (3) (5).
The 5% dextrose minimizes the hemolytic action of guaifenesin alone and reduces the tendency to form thrombi at the intravenous injection site (6). Guaifenesin appears safe when administered to pregnant mares (7) and in human studies did not pass the placental barrier (2).
Metabolism and excretion of guaifenesin is not well understood. Oxidation products are excreted in the urine as a glycuronimide.
GuaifenJect Indications
For intravenous use as a skeletal muscle relaxant for horses.
Contraindications
Do not administer physostigmire to horses receiving GuaifenJect.
WARNINGS Not to be used in horses intended for food.
Destroy partially used vials.
PRECAUTIONS Avoid perivascular leakage to prevent local tissue irritation.
If used for prolonged periods, such as in tetanus, serum hemoglobin levels should be monitored.
Oxygen or sustained artificial respiration facilities should be available when the drug is employed.
Additional care should be employed when administering the drug to anemic or hypovolemic animals with cardiac or respiratory problems.
Extreme care should be exercised at all times in handling this product to prevent the introduction of microbial contamination.
DOSAGE AND ADMINISTRATION For intravenous use only. Administer rapidly with a positive pressure system or gravity flow using a 12 to 14 gauge needle at the dose rate of 1 mL/pound of body weight. From a standing position the patient will begin to relax and gradually fall when approximately 1/2 the total dose has been given. Continue the remaining calculated dose for complete relaxation. Average duration of muscle relaxation is 10-25 minutes. For continued relaxation time, additional GuaifenJect may be administered as determined by the veterinarian at a rate necessary to achieve the desired plane of relaxation. Recovery is usually smooth and uneventful with the horse regaining an upright position usually within 45 minutes after the medication is discontinued.
HOW SUPPLIED 1,000 mL sterile, single dose plastic vial.
Store between 2° and 30°C (36° and 86°F).
Do not freeze.
REFERENCES
1. Schebitz, H. and Tronick, R.: Berliner und Munchener Tierarztliche, Wochenschrift, 77 (5) (March 1964), pp. 93-97.
2. Westhues, M. and Fritsch, R.: "Animal Anesthesia-General", J.P. Lippincott Co., Philadelphia, PA, 1961 (Translation 1965).
3. Truitt, E.B., Jr. and Patterson, R.B.: Proc. Soc. Exp. Bio. Med., 95 ( 1957) pp. 422-424.
4. Heath, R.B. and Gabel, A.A.: JAVMA, 157, No. 11 (Dec. 1, 1970), pp. 486-494.
5. Garton, G.A. and Williams, R.T.: Biochem, J., 43 (1948), pp. 206-211.
6. Ginzel, K.H., Leupold-Lowenthal, H. and Weis, W.: Weiner Medizinische Wochenschrift, 99 (1949), pp. 299-300.
7. Davis, L.E. and Wolff, W. A.: Am. J. Vet. Res., 31, No. 3 (March 1970), pp. 469-473.
FOR ANIMAL USE ONLY
NOT FOR HUMAN USE
KEEP OUT OF THE REACH OF CHILDREN
600024
Rev. 5-98
Manufactured for: HENRY SCHEIN ANIMAL HEALTH, DUBLIN, OHIO 43017
CPN: 10820884
400 METRO PLACE NORTH, DUBLIN, OH, 43017-7545
| Telephone: | 614-761-9095 | |
| Toll-Free: | 1-855-724-3461 |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
