Muricin (mupirocin) Ointment 2%
This treatment applies to the following species: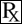 Company: Dechra
Company: Dechra
mupirocin
ANADA # 200-418. Approved by FDA.
For dermatologic use on dogs.
Muricin (mupirocin) Ointment 2% Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
Each gram of Muricin® ointment contains 20 mg of mupirocin in a bland, water-washable ointment base consisting of polyethylene glycol 400 and polyethylene glycol 3350 (polyethylene glycol ointment, NF). Mupirocin is a naturally-occurring, broadspectrum antibiotic. The chemical name is (E) - (2S,3R,4R,5S) - 5 - [(2S,3S,4S,5S) - 2,3 - Epoxy - 5 - hydroxy - 4 - methylhexyl]tetrahydro - 3,4 - dihydroxy - β - methyl - 2H - pyran - 2 - crotonic acid, ester with 9-hydroxynonanoic acid. The chemical structure is:
Clinical Pharmacology
Mupirocin is a chemical entity produced by fermentation of the organism Pseudomonas fluorescens. Mupirocin inhibits bacterial protein synthesis by reversibly and specifically binding to bacterial isoleucyl transfer-RNA synthetase. Due to this mode of action, mupirocin shows no cross resistance with chloramphenicol, erythromycin, gentamicin, lincomycin, neomycin, novobiocin, penicillin, streptomycin, and tetracycline. Mupirocin is an antimicrobial agent that inhibits the growth of gram-positive and gram-negative bacteria. Bacteria susceptible to the action of mupirocin in vitro include the aerobic isolates of Staphylococcus aureus (including methicillin-resistant strains and β-lactamase-producing strains), Staphylococcus intermedius, Staphylococcus epidermidis, other coagulase positive or negative Staphylococci, α-hemolytic Streptococci, β group A Streptococci (including S. pyogenes), other β Streptococci (including S. agalactiae), group D Streptococci (including S. faecalis and S. faecium), group Viridans Streptococci, Streptococcus pneumoniae, Corynebacterium hofmanii, Bacillus subtilis, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Proteus vulgaris, Enterobacter cloacae, Enterobacter aerogenes, Citrobacter freundii, Hemophilus influenzae (including β-lactamase-producing strains), Neisseria gonorrheae (including β-lactamase-producing strains), Neisseria meningitidis, Branhamella catarrhalis and Pasteurella multocida, and the anaerobic isolates of Peptostreptococcus anaerobius, Clostridium difficile, and Clostridium sporogenes.Clinical significance of the in vitro data is unknown except for susceptible strains of Staphylococcus aureus and Staphylococcus intermedius.
INDICATIONS FOR USE: Muricin® ointment is indicated for the topical treatment of canine bacterial infections of the skin, including superficial pyoderma, caused by susceptible strains of Staphylococcus aureus and Staphylococcus intermedius.
Contraindications
This drug is contraindicated in animals with a history of sensitivity reactions to any of its components.
Warnings
Because of the potential hazard of nephrotoxicity due to the polyethylene glycol content of the base, care should be exercised when using this product in treating extensive deep lesions where absorption of large quantities of polyethylene glycol is possible.Safety of use in pregnant or breeding animals has not been determined.
Muricin® ointment is not for ophthalmic use.
Adverse Reactions
No adverse reactions have been reported with this product. If a skin reaction such as irritation should occur, treatment should be discontinued and appropriate therapy instituted.Dosage and Administration
Prior to treatment, the lesion should be cleansed. Muricin® ointment should be applied to the affected area twice a day. Apply a sufficient amount of ointment to completely cover the infected area. Maximum duration of treatment should not exceed 30 days.
1. Remove entire Applicator Assembly, puncture tube with white portion of the cap.

2. Replace entire Applicator Assembly.
3. To dispense, remove the white portion of the cap.
4. After use replace white cap to close.
IMPORTANT: The opening of this product is covered by a metal tamper-resistant seal. If this seal has been punctured or is not visible, do not use and return product to place of purchase.
How Supplied
Muricin® ointment is supplied in 15-gram tubes.NDC 17033-420-15.
Store between 15° and 30°C (59° and 86°F).
Keep Out of Reach of Children.
MANUFACTURED FOR: Dechra Veterinary Products, Overland Park, KS 66211
I420 DECHRA R1/08
#131
CPN: 14590490
7015 COLLEGE BLVD., STE. 525, OVERLAND PARK, KS, 66211
| Telephone: | 913-327-0015 | |
| Toll-Free: | 888-337-0929 | |
| Technical Support: | 866-933-2472 | |
| Fax: | 913-327-0016 | |
| Website: | www.dechra-us.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
