SUPRELORIN 4.7 mg Implant (Deslorelin Implant) for Dogs (Canada)
This treatment applies to the following species: Company: Virbac
Company: Virbac
Veterinary Use Only
DIN 02526689
Read the entire Package Insert before use.
Description
SUPRELORIN 4.7 mg Implant is a white to pale yellow cylindrical implant containing 4.7 mg deslorelin (as deslorelin acetate).
DESLORELIN:
i) Deslorelin chemical structure:
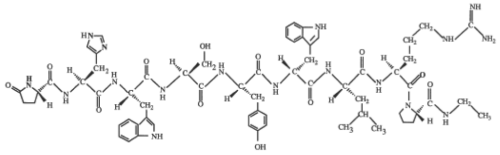
ii) Amino acid sequence: pGlu-His-Trp-Ser-Tyr-D-Trp-Leu-Arg-Pro-NHEt
iii) Molecular formula: C64H83N17O12 · xCH3COOH · yH2O
SUPRELORIN 4.7 mg Implant (Deslorelin Implant) for Dogs Indications
To temporarily suppress testosterone concentrations in healthy, entire (intact), sexually mature male dogs to cause infertility.
Dosage and Administration
One implant should be administered subcutaneously between the shoulder blades of the dog. Administer one implant only, irrespective of the size of the dog.
Testosterone suppression and infertility are not immediate. Testosterone concentrations can be monitored to aid in the determination of the period of infertility. Testosterone concentrations less than 1.4 nmol/L (0.4 ng/mL) are associated with infertility.
Do not use the product if the foil pouch is broken.
Disinfection of the implantation site should be undertaken prior to implantation to prevent infection. Select the implant site by locating the area of the back midway between the shoulder blades. Avoid injection of the implant into fat, as release of the active substance might be impaired in areas of low vascularization. If the hair is long, a small area may be clipped, if required.
1. Remove the Luer Lock cap from the implanter.
2. Attach the actuator to the implanter using the Luer Lock connection.
3. Lift the loose skin between the shoulder blades. Insert the entire length of the needle subcutaneously.
4. Fully depress the actuator plunger and, at the same time, slowly withdraw the needle.
5. Press the skin at the insertion site as the needle is withdrawn, and maintain pressure for 30 seconds.
6. Examine the syringe and needle to ascertain that the implant has not remained within the syringe or needle, and that the spacer is visible. It may be possible to palpate the implant in situ.
PLEASE NOTE: DO NOT THROW THE ACTUATOR SYRINGE AWAY. There is only 1 actuator syringe per box. It will be needed to administer the remaining implant.
Contraindications
Do not use in cryptorchid dogs.
CAUTIONS:
Although testosterone suppression is associated with infertility, the period of infertility associated with testosterone suppression varies depending on the individual dog. Infertility would be expected 6 to 9 weeks after implantation and persist for at least 156 days (See EFFICACY).
Dogs should reach puberty before treatment with SUPRELORIN 4.7 mg Implant is initiated. Delayed physeal closures, and non palpable testicles may be observed if SUPRELORIN 4.7 mg Implant is administered to pre-pubertal dogs.
Testosterone concentrations may increase in the first weeks after implantation before testosterone suppression occurs.
An increase in aggressive behaviour may be seen initially after SUPRELORIN 4.7 mg Implant is implanted and again when testosterone concentrations begin to increase as the efficacy of the implant is decreasing.
Dogs that mate may cause pregnancy. Treated dogs should be kept away from bitches in heat within the first 6 weeks after initial treatment.
Should a treated dog mate with a bitch after treatment, appropriate measures should be taken to rule out pregnancy.
The biocompatible implant does not require removal.
The ability of dogs to sire offspring following their return to normal plasma testosterone levels, after the administration of SUPRELORIN 4.7 mg Implant has not been investigated.
After implantation, signs associated with hormonal down-regulation can be observed, such as, decrease in testicle size, reduced activity, and weight gain. In very rare cases, a testicle may be able to ascend through the inguinal ring.
Data demonstrating the complete reversibility of clinical effects (reduced testicular size, reduced ejaculation volume, reduced sperm count and reduced libido) including fertility are limited.
If loss of the implant is suspected (e.g. by absence of reduction of scrotal circumference), this can be confirmed by observing no reduction in plasma testosterone levels after 6 weeks from the suspected date of loss. Testosterone concentration should decrease under correct implantation.
Warnings
Pregnant women should not administer the product. A GnRH analogue has been shown to be fetotoxic in laboratory animals.
When administering the product, care should be taken to avoid accidental self-injection by ensuring that the animals are suitably restrained and the application needle is shielded until the moment of implantation. Wash hands after handling.
In case of accidental self-injection, seek medical attention immediately, with a view to having the implant removed. Show the product label to the physician.
Keep out of reach of children.
Precaution
Do not use in animals intended for breeding. The safe use of this product has not been evaluated in pregnant or lactating ferrets.
Adverse Reactions
Although all adverse reactions are not reported, the following information is based on voluntary post-approval drug experience reporting. It is generally recognized that this results in significant under-reporting. The adverse events listed here reflect reporting and not necessarily causality. Adverse reactions are listed by body system in decreasing order of frequency, and have been reported very rarely (<1/10,000 treated animals):
Systemic disorders: polydipsia, anorexia, lethargy
Renal and urinary disorders: urinary incontinence, polyuria
Behavioural disorders: sexual disorders NOS, anxiety
Skin and appendages disorders: alopecia
Application site disorders: implant site swelling, implant site reaction NOS
Rarely (between 1-10/10,000 treated animals), lack of efficacy has been reported after the use of this product. In the majority of cases a lack of reduction of testicle size was reported and/or a bitch was mated.
In very rare cases (<1/10,000 treated animals), a transient behavioural change has been reported with development of aggression. Aggression has been noted towards other dogs, as well as towards humans.
In humans and animals, testosterone modulates seizure susceptibility. On very rare occasions transient occurrence of seizure has been reported after implantation. In some cases, the dog had displayed epileptic seizure prior to the implant administration or was diagnosed as suffering from epilepsy.
Moderate swelling at the implant site was observed for 14 days during safety/efficacy studies.
During the treatment period, rare clinical effects have been reported: hair coat disorders (e.g. hair loss, alopecia, hair modification), urinary incontinence. Signs associated with hormonal down-regulation can be observed, such as, decrease in testicle size, reduced activity, weight gain. In very rare cases, a testicle may be able to ascend the inguinal ring.
In very rare cases there has been transitory increased sexual interest, increased testicle size and testicular pain immediately after implantation. These signs resolved without treatment.
Clinical Pharmacology
Pharmacodynamics: Deslorelin is a GnRH agonist, which acts by down regulating the GnRH receptors of the anterior pituitary, thus reducing the release of the gonadotrophins LH and FSH. FSH maintains early spermatogenesis, whilst LH promotes the release of testosterone. Testosterone is essential for the completion of spermatogenesis, which takes seven to 10 weeks in total. The clinical effects of deslorelin are varied, but in essence they involve the reduction of semen quality and quantity. More specifically, these effects include reduced semen volume, poor sperm count, increased rates of morphological defects in sperm and reduced motility, reduced libido and can also reduce testicular size. These effects are attributable to the sustained reduction, by deslorelin implant, of plasma testosterone levels to below the average lower normal limit seen in dogs.
Pharmacokinetics: Deslorelin, like the other GnRH analogues is a peptide hormone and, consequently, it will be largely broken down by digestive enzymes and acid hydrolysis following oral ingestion, and it has been shown that the potency of GnRH analogues is several orders of magnitude lower following oral administration than following parenteral injection. Although more stable than GnRH itself, GnRH analogues are rapidly absorbed and rapidly eliminated, predominantly by metabolism to inactive peptide fragments, following parenteral administration.
Six intact male beagle dogs aged 22 months were treated subcutaneously with one SUPRELORIN 4.7 mg Implant. The pharmacokinetics of deslorelin plasma concentrations were evaluated along with the pharmacodynamics effects over the 441 day study. The highest mean plasma concentration (Cmax at 21,787 ± 9,229 pg/mL) was observed 1 hour post implantation (Tmax) and rapidly decreased by 95% in the first 24 hours after implantation. From study day 2 post implantation, there was a slow but continuous decline in plasma deslorelin concentration in all dogs.
Deslorelin was non-quantifiable (<4 pg/mL) in 5/6 dogs starting between study day 196 and study day 414. The sixth dog had a deslorelin concentration of 4.28 pg/mL at the end of the study (day 441). Inter-individual variability in plasma deslorelin concentrations was high. The mean Area Under the Curve inf was 34,004 ± 11,149 pg/mL*day (coefficient of variation 33%) with a range of 22,632 to 50,566 pg/mL*day.
The pharmacodynamic parameter testosterone was evaluated in the pharmacokinetic study described above. Before treatment, the mean testosterone serum concentration was 6.6 ± 4.26 nmol/L. After administration there was an initial increase in mean testosterone concentrations to 21.1 ± 7.5 nmol/L, Testosterone then decreased from study day 8. From study day 28 to study day 168, mean values of testosterone ranged from 0.13 to 0.45 nmol/L. On study day 224, mean testosterone values increased to 5.2 ± 8.8 nmol/L. After study day 224, testosterone concentrations returned to baseline values until the end of the study. The dog with the shortest duration of detectable deslorelin concentrations did not have the shortest period of suppressed testosterone concentrations. The duration of effect does not appear to be directly related to the plasma deslorelin concentration.
EFFICACY:
Seventy-two (72) healthy adult male dogs with two descended testicles were enrolled in a 184 day randomized laboratory study conducted in China. There were 3 test article treated groups (<10 kg, 10-25 kg, >25 kg) and 3 control groups (<10 kg, 10-25 kg, >25 kg) with 20 dogs in each test article treated group and 4 dogs in each control group. On study day 0, each dog in the test article group was subcutaneously implanted with SUPRELORIN 4.7 mg Implant, whereas the control group dogs, received no treatment. From study day 0 until the end of the study, serum testosterone and scrotal circumferences were measured regularly. Semen was collected from each dog on study day -5 ± 1 day and then on study day 180. In addition, the safety of SUPRELORIN 4.7 mg Implant was assessed by complete blood counts and serum biochemistry on study days 0 and 184 and monitoring for adverse events during the study.
The primary study endpoint was the change in serum testosterone concentration and the secondary study endpoints were scrotal circumference, semen analysis and sexual behaviour. When serum testosterone is continuously suppressed reproductive function is inhibited.
Serum testosterone concentrations in all three groups of SUPRELORIN 4.7 mg Implant treated dogs was significantly increased 1 hour after implantation and remained increased until study day 14. By study day 28.5 mean serum testosterone concentrations had decreased to below 0.087 nmol/L (0.025 ng/mL) (the limit of detection). By study day 46, 68% of dogs had serum concentrations < 0.087 nmol/L (0.025 ng/mL). On study day 63, 95% of dogs had serum testosterone concentrations less than < 0.087 nmol/L (0.025 ng/mL).
Serum testosterone concentrations of less than < 0.087 nmol/L were maintained until the end of the study (184 days) in 56/60 treated dogs. The mean period of testosterone suppression below the limit of detection was 155.7 days in the 184 day study. There were statistically significant differences in testosterone concentrations over time in dogs in test article groups, but there was no statistical difference among the three test article treated groups. There were statistically significant differences in testosterone concentrations between SUPRELORIN 4.7 mg Implant treated groups and the control groups of dogs.
In test article treated groups, the scrotal circumference decreased significantly after treatment with SUPRELORIN 4.7 mg Implant, and remained decreased until the end of the study, with statistically significant changes in the scrotal circumference before and after implantation and compared to the control dogs. On study day -5 ± 1, semen was collected from 63% of dogs in test article treated groups, and from 50% of dogs in the control groups. At the end of the study, all dogs in the SUPRELORIN 4.7 mg Implant treated groups could no longer ejaculate. Semen was successfully collected from 33.3% (4/12) of dogs in control groups. The success of semen collection on study day 184 was significantly different between the treatment and control groups compared to study day -5 ± 1 when there was no significant difference between the groups. There was no significant difference within the control groups between study day -5 ± 1 and study day 184.
There was no statistically significant difference in the effect between large (>25 kg), medium (10-25 kg), and small (<10 kg) breed dogs when the SUPRELORIN 4.7 mg Implant was administered at the recommended dose of one subcutaneous implant per dog.
Deslorelin acetate implants (SUPRELORIN 4.7 mg Implant) caused implantation site reactions in 4/60 dogs (6.7%): two dogs had skin ulcerations, 1 dog had mild erythema and swelling and 1 dog had erythema. The adverse events resolved in all the dogs within 9 days. No other drug related adverse events were observed. The results of routine blood tests and biochemical examinations were not affected by SUPRELORIN 4.7 mg Implant.
SAFETY:
Thirty-two dogs: 16 intact males and 16 intact females, approximately 12 weeks old at first treatment, were randomized into four treatment groups with four animals per sex, per group. Group 1 (0x dose - Placebo, untreated); Group 2 (1x dose); Group 3 (3x dose); and Group 4 (5x dose). The test article and control article were administered on Day 0, again at approximately six months (Day 182) and finally at approximately 12 months (Day 364) after the first treatment. The test (SUPRELORIN 4.7 mg Implant) or control (saline) articles were injected or implanted subcutaneously between the shoulder blades.
Animals were observed twice daily from the acclimation period (Day -14) to the end of the in-life part of the study (Day 394). Clinical observations, food consumption, body weights, rectal body temperature, physical exams, neurological exams and injection site observations were performed over the course of the study. Radiographs (growth plate assessment) and DEXA scans (bone mineral density evaluation) were also performed. Specimens were collected throughout the study: blood for deslorelin analysis, testosterone for the male dogs, hematology and biochemical analyses, urine for urinalysis. Following euthanasia and necropsy, organ weights, bone marrow smears and histopathology were done.
Clinical adverse events observed included: hair loss, ocular discharge, umbilical hernia, scratching, excitedness, aggressiveness during handling, cherry eye, grand mal seizures, redness and scabbing of the skin, lumps under the injection sites, undescended testicles, diarrhea and blood at rectum.
Injection site observations included bleeding, redness, swelling occasional irritation and lumps near the injection site.
Undescended testicles were recorded for males treated with the test article and were correlated with testicular degeneration and epididymal atrophy. Undescended testicles in the males was an expected pharmacological effect of the test article.
Grand mal seizures were observed in one male and one female dog in the 5x group. The reason for the seizures was not determined.
There were no test article related effects in the physical or neurological examinations and no effects on body weight, food consumption, rectal temperature, heart rate or respiratory rate.
Treatment related changes in clinical pathology findings included increased hematocrit, hemoglobin and red blood cell counts in all treatment groups (1x, 3x and 5x) and alkaline phosphatase was increased in all treatment groups beginning on day 28 through day 392. Cholesterol was increased in the 3x and 5x treated dogs. These effects were considered non-toxicologically relevant. There was no test article related changes in the urinalysis parameters.
Bone mineral density was decreased at day 390 in males in the 5x group. A delay in the closure of the proximal physis of the humerus in all treatment groups and a delay in the closure of the distal physis of the femur in males at the 3x and 5x group was observed. These effects were considered to be test article related but non-adverse in this study.
Deslorelin acetate plasma levels in treated groups increased proportionally to dose from Day 181 through Day 189 and the level of deslorelin in the blood stayed near this concentration before a sharp decrease from Day 210 through Day 363. No accumulation of deslorelin from a previous implantation was observed.
Treatment-related changes in organ weights were limited to epididymides, testes, ovaries, adrenals, and kidneys. Changes in reproductive organ weights were a pharmacological effect of the test article. Adrenal weight was statistically higher than control in males in the 5x group and kidney weights in males was statistically lower than control at 1x, 3x and 5x. There were no associated histopathological or biochemical findings associated with the organ weight reductions.
Microscopic atrophy of the reproductive organs in the treated males included testicular degeneration, prostate and epididymal atrophy. Female reproductive organs were also atrophied. Cellulitis associated with the implant was observed. These findings were expected with the test article.
Subcutaneous administration of deslorelin acetate 4.7 mg administered to dogs at 1x, 3x and 5x dose was associated with treatment-related injection site reactions and expected atrophy of male and female reproductive organs.
Storage
Store in a refrigerator (2°C - 8°C). Do not freeze. Do not use this veterinary medicinal product after the expiry date which is stated on the carton.
PRESENTATION:
Package sizes are 2 and 5. Not all pack sizes may be marketed.
Virbac AH, Inc., PO Box 162059, Fort Worth, Texas, United States 76161
Imported and distributed by Virbac Canada, Inc., 231 Shearson Crescent, Suite 209, Cambridge, ON, N1T 1J5. 1-800-338-3659
SUPRELORIN is a registered trademark of Virbac (Australia) Pty Ltd. © 2022 Virbac Corporation. All Rights Reserved.
44406
84297101
|
Net: |
|
|
Box with 2 implants preloaded implanters and 1 actuator. |
84297001 P-2001-CA-1 |
CPN: 1177122.0
209-231 SHEARSON CRESCENT, CAMBRIDGE, ON, N1T 1J5
| Toll-Free: | 866-458-3350 | |
| Fax: | 844-458-4004 | |
| Website: | https://ca.virbac.com/ |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
