Torphadine 10 mg/mL (Canada)
This treatment applies to the following species: Company: Dechra
Company: Dechra
Butorphanol Tartrate Injection, Mfr. Std.
Sterile
Veterinary Use Only
DIN 02496402
Description
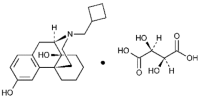
Butorphanol tartrate is a totally synthetic, centrally acting, narcotic agonist-antagonist analgesic with potent antitussive activity. It is a member of the phenanthrene series. The chemical name is Morphinan-3, 14-diol, 17-(cyclobutylmethyl)-, (-), [S- (R*,R*)]- 2,3-dihydroxybutanedioate (1:1) (salt). It is a white, crystalline, water soluble substance having a molecular weight of 477.55; its molecular formula is C21H29NO2 • C4H6O6.
Chemical structure:
Active Ingredient
Each mL of Torphadine contains 10 mg butorphanol base (as butorphanol tartrate).
Preservative: Benzethonium chloride 0.1 mg/mL
Torphadine 10 mg/mL Indications
Torphadine (butorphanol tartrate injection) is indicated for the relief of pain associated with colic in adult horses and yearlings. Clinical studies in the horse have shown that butorphanol alleviated abdominal pain associated with torsion, impaction, intussusception, spasmodic and tympanic colic, and postpartum pain.
Torphadine 10 mg/mL Dosage And Administration
The recommended dosage in the horse is 0.1 mg butorphanol per kilogram of body weight by intravenous injection. This is equivalent to 5 mL of Torphadine (butorphanol tartrate injection) for each 454 kg body weight. Dose may be repeated as required every 3 hours for a maximum of 48 hours. Pre-clinical model studies and clinical field trials in horses demonstrate that the analgesic effects of butorphanol are seen within 30 minutes following injection and persist for about 60-90 minutes.
Torphadine 10 mg/mL Cautions
For use in horses only.
Torphadine (butorphanol tartrate injection), a potent analgesic, should be used with caution with other sedative or analgesic drugs as these are likely to produce additive effects.
Like other centrally acting compounds there may be a variation in response to Torphadine (butorphanol tartrate injection) between different equine breeds and between individual horses.
There are no well-controlled studies using butorphanol in breeding horses, weanlings, and foals. Therefore the drug should not be used in these groups.
Warnings
This drug is not to be administered to horses that are to be slaughtered for use in food.
Keep out of reach of children.
Adverse Reactions
In clinical trials in horses, the most commonly observed side effect was slight ataxia which lasted 3 to 10 minutes. Marked ataxia was reported in 1.5% of the 327 horses treated. Mild sedation was reported in 9% of the horses.
Extensive overdosing may cause signs of narcosis as outlined under «Equine Pharmacology», Acute equine studies.
Clinical Pharmacology
Comparative pharmacology:
In animals, butorphanol has been demonstrated to be 4 to 30 times more potent than morphine and pentazocine (Talwin®-Winthrop) respectively.1 In humans, butorphanol has been shown to have 5 to 7 times the analgesic activity of morphine and 20 times that of pentazocine.2,3 Butorphanol has 15 to 20 times the oral antitussive activity of codeine or dextromethorphan in dogs and guinea pigs.4
As an antagonist, butorphanol is approximately equivalent to nalorphine and 30 times more potent than pentazocine.1
Cardiopulmonary depressant effects are minimal after treatment with butorphanol as demonstrated in dogs5, humans6,7 and horses.8 Unlike classical narcotic agonist analgesics which are associated with decreases in blood pressure, reduction in heart rate, and concomitant release of histamine, butorphanol does not cause histamine release.1 Furthermore, the cardiopulmonary effects of butorphanol are not distinctly dosage related but rather reach a ceiling effect beyond which further dosage increases result in relatively less effects.
Reproduction:
Studies performed in mice and rabbits revealed no evidence of impaired fertility or harm to the fetus due to butorphanol tartrate. In the female rat, parenteral administration was associated with increased nervousness and a decreased care for the newborn, resulting in a decreased survival rate of the newborn. This nervousness was seen only in the rat species.
Equine pharmacology:
Following intravenous injection in horses, butorphanol is largely eliminated from the blood within 3 to 4 hours. The drug is extensively metabolized in the liver and excreted in the urine.
In ponies, butorphanol given intramuscularly at a dosage of 0.22 mg/kg, was shown to alleviate experimentally induced visceral pain for about 4 hours.9
In horses, intravenous dosages of butorphanol ranging from 0.05 to 0.4 mg/kg were shown to be effective in alleviating visceral and superficial pain for at least four hours.
A definite dosage-response relationship was detected in that butorphanol dosage of 0.1 mg/kg was more effective than 0.05 mg/kg but not different from 0.2 mg/kg in alleviating deep abdominal pain.
Acute equine studies:
Rapid intravenous, administration of butorphanol at a dosage of 2.0 mg/kg (20 times the recommended dosage) to a previously unmedicated horse resulted in a brief episode of inability to stand, muscle fasciculation, a convulsive seizure of 6 seconds duration, and recovery within three minutes.
The same dosage administered after 10 successive daily 1.0 mg/kg dosages of butorphanol resulted only in transient sedative effects. During the 10 day course of administration at 1.0 mg/kg (10 times the recommended use level) in two horses, the only detectable drug effects were transient behavioural changes typical of narcotic agonist activity. These included muscle fasciculation about the head and neck, dysphoria, lateral nystagmus, ataxia, and salivation. Repeated administration of butorphanol at 1.0 mg/kg (10 times the recommended dose) every four hours for 48 hours caused constipation in one of two horses.
Subacute equine studies:
Horses were found to tolerate butorphanol given intravenously at doses of 0.1, 0.3 and 0.5 mg/kg every 4 hours for 48 hours followed by once daily injections for a total of 21 days. The only detectable drug effects were slight transient ataxia observed occasionally in the high dosage group. No clinical, laboratory, or gross or histopathologic evidence of any butorphanol-related toxicity was encountered in the horses.
Storage
Store below 30°C. Protect from freezing. Keep the vial in the carton to protect from light. Contents should be used within 28 days after first dose is removed.
HOW SUPPLIED
Clear glass vials of 10 or 20 mL.
REFERENCES
1. Pircio, A.W., et al: The Pharmacology of Butorphanol, a 3, 14-Dihydroxymorphinan Narcotic Antagonist Analgesic. Arch. Int. Pharmacodyn. Ther. 220(2): 231-257, 1976.
2. Dobkin, A.B., et al: Butorphanol and Pentazocine in Patients with severe Postoperative pain. Clin. Pharmacol. Ther. 18: 547-553, 1975.
3. Gilbert, M.S., et al: Intramuscular Butorphanol and Meperidine in Postoperative pain. Clin. Pharmacol. Ther. 20: 359-364, 1976.
4. Cavanagh, R.L., et al: Antitussive Properties of Butorphanol. Arch. Int. Pharmacodyn. Ther. 220: 258-268, 1976.
5. Schurig, J.E., et al: Effect of Butorphanol and Morphine on Pulmonary Mechanics, Arterial Blood Pressure, and Venous Plasma Histamine in the Anesthetized Dog. Arch. Int. Pharmacodyn. Ther. 233: 296-304, 1978.
6. Nagashmina, H., et al: Respiratory and Circulatory Effects of Intravenous Butorphanol and Morphine. Clin. Pharm. Ther. 19: 738-745, 1976.
7. Popio, K.A., et al: Hemodynamic and Respiratory Effects of Morphine and Butorphanol. Clin. Pharm. Ther. 23: 281-287, 1978.
8. Robertson, J.T., et al: Cardiopulmonary Effects of Butorphanol Tartrate in Horses. Am. J. Vet. Res. 42: 41-44, 1981.
9. Kalpravidh, M., et al: Effects of Butorphanol, Flunixin, Levorphanol, Morphine, and Xylazine in Ponies. Am. J. Vet. Res. 45: 217-223, 1984.
Manufactured by:
Dechra Regulatory B.V., Handelsweg 25, 5531 AE Bladel, The Netherlands
Distributed and imported by:
Dechra Veterinary Products Inc., 1 Holiday Avenue, East Tower, Suite 345, Pointe-Claire, Québec, H9R 5N3, Canada
CPN: 1786060.1
1 HOLIDAY AVE., EAST TOWER SUITE 345, POINT-CLAIRE, QC, H9R 5N3
| Toll-Free: | 855-332-9334 | |
| Technical Services: | 855-332-9334 Option 1 | |
| Technical Services Email: | technical.ca@dechra.com | |
| Website: | www.dechra.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
