VETSPON
This treatment applies to the following species: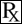 Company: Ferrosan Medical Devices
Company: Ferrosan Medical Devices
VETSPON® Flex
Absorbable Hemostatic Gelatin Sponge, U.S.P.
VETSPON® Dental
Absorbable Hemostatic Gelatin Sponge, U.S.P.
VETSPON Caution
Federal (U.S.A.) law restricts this device to sale by or on the order of a licensed veterinarian.
Description
VETSPON® Absorbable Hemostatic Gelatin Sponges are sterile, water-insoluble, malleable, absorbable porcine gelatin sponges intended for hemostasis by applying to a bleeding surface. They are off-white and porous in appearance.
VETSPON Indications For Use
VETSPON®, used dry or saturated with sterile sodium chloride solution, is indicated for surgical procedures (except ophthalmic) for hemostasis, when control of capillary or small venous or arteriolar bleeding (oozing bleeding) by pressure, ligature or other conventional procedures is ineffective or impractical. Although not necessary, VETSPON® can be used with thrombin to achieve hemostasis.
Contraindications
Do not use VETSPON® in closure of skin incisions because it may interfere with the healing of skin edges. This interference is due to mechanical interposition of gelatin and is not secondary to intrinsic interference with wound healing.
Do not use VETSPON® in intravascular compartments because of the risk of embolization.
Do not use VETSPON® in patients with known allergies to porcine collagen or gelatin.
Warnings
VETSPON® is not intended as a substitute for meticulous surgical technique and the proper application of ligatures or other conventional procedures for hemostasis.
VETSPON® should not be used in the presence of infection. VETSPON® should be used with caution in contaminated areas of the body. If signs of infection or abscess develop where VETSPON® has been positioned, surgery may be necessary to remove the infected material and allow drainage.
VETSPON® should not be used in instances of pumping arterial hemorrhage. It should not be used where blood or other fluids have pooled or in cases where the point of hemorrhage is submerged. VETSPON® will not act as a tampon or plug in a bleeding site, nor will it close off an area of blood collecting behind a tampon.
VETSPON® should be removed if possible once hemostasis has been achieved because of the possibility of dislodgment of the device or compression of other nearby anatomic structures.
VETSPON® should be removed from the site of application when used in, around, near foramina in bone, areas of bony confine, the spinal cord, and/or the optic nerve and chiasm.
The safety and effectiveness of VETSPON® for use in ophthalmic procedures has not been established.
VETSPON® should not be used for controlling post-partum bleeding.
Precautions
Although absorbable hemostasis products have been used in veterinary medicine, safe and effective use of this product has not been proven through randomized, prospective controlled clinical studies on animals.
VETSPON® is supplied as a sterile product and cannot be resterilized. Discard unused portions of open envelopes or blisters of VETSPON®.
When placed into cavities or closed tissue spaces, use minimal preliminary compression and avoid overpacking of VETSPON® into the cavity. VETSPON® may swell to its original size on absorbing fluids, creating the potential for nerve damage.
While packing a cavity for hemostasis is sometimes surgically indicated, VETSPON® should only be used in this manner if excess product not needed to maintain hemostasis is removed.
Use only the minimum amount of VETSPON® needed to achieve hemostasis. Once hemostasis is achieved, any excess VETSPON® should be carefully removed.
VETSPON® should not be used in conjunction with methyl methacrylate adhesives. In humans, microfibrillar collagen has been reported to reduce the strength of methyl methacrylate adhesives used to attach prosthetic devices to bone surfaces.
VETSPON® should not be used for the primary treatment of coagulation disorders.
The safety and effectiveness of the combined use of VETSPON® with other agents such as topical thrombin, antibiotic solution or antibiotic powder have not been evaluated in controlled clinical trials and therefore cannot be recommended. If in the veterinarian’s judgment, concurrent use of topical thrombin or other agents is medically advisable, consult the product literature for that agent for complete prescribing information.
The safety and effectiveness for use in urological procedures have not been established through a randomized clinical study.
In urological procedures, VETSPON® should not be left in the renal pelvis or ureters to eliminate the potential foci for calculus formation.
Adverse Events
For Technical Assistance please call 1-888-545-5973.
Gelatin-based hemostatic agents may serve as a nidus for infection and abscess formation and have been reported to potentiate bacterial growth.
Although controlled studies using VETSPON® in animals have not been conducted, in general, the following adverse events have been reported with the use of absorbable porcine gelatin-based hemostatic agents in humans: Infection, pain, fever, blindness, paresis, bladder and bowel dysfunction, and granulomas.
In some cases these events were associated with device migration. Hematoma, encapsulation and foreign body reactions also have been reported after use in humans.
To report adverse effects, access medical information, or obtain additional product information call 1-888-545-5973.
Storage And Handling
VETSPON® should be stored dry at controlled room temperature 15°-30°C (59°-86°F).
VETSPON® should be used as soon as the package is opened. Discard partially used packages. Do not attempt to resterilize partially used units.
Directions For Use
Before using, inspect the package for signs of damage. If the package is damaged or wet, sterility cannot be assured and the contents should not be used. Use sterile technique to remove VETSPON® from its packaging.
Cut the sheet to the desired size. Use only the minimum amount necessary to achieve hemostasis. This piece of VETSPON® can be applied to the bleeding site either dry or saturated with sterile isotonic sodium chloride solution (sterile saline). Open packages of VETSPON® should be discarded, since they are not intended for reuse and/or resterilization.
When used in appropriate amounts the sponge is absorbed completely within 4 to 6 weeks. In an animal implantation study, tissue reactions were classified as negligible when observed macroscopically and moderate when observed microscopically. When applied to bleeding mucosal regions, VETSPON® liquefies within 2 to 5 days.
A) Dry Use:
1. Cut the VETSPON® sponge or cube to desired size and shape.
2. Manually compress the sponge prior to applying to the bleeding site.
3. Hold VETSPON® in place with moderate pressure until hemostasis is achieved.
4. If desired, remove excess VETSPON® upon achieving hemostasis by gently irrigating the site with sterile saline solution to completely wet the sponge.
5. The portion of the VETSPON® that is adhering to the bleeding site may be left in situ. Use only the amount required to achieve hemostasis and remove any excess.
B) Use With Sterile Saline Preparation:
1. Cut the VETSPON® sponge or cube to desired size and shape.
2. Immerse the VETSPON® in the saline solution.
3. Withdraw the sponge and squeeze between gloved fingers to expel air bubbles.
4. Return sponge to the solution. The VETSPON® should promptly return to its original size and shape in the solution. If it does not, remove it from the solution and vigorously knead it between gloved fingers until all air is expelled and it returns to its original size and shape when placed in the solution.
5. Blot the sponge to desired dampness on gauze before applying to the bleeding site.
6. Hold the VETSPON® in place with gauze using moderate pressure until hemostasis is achieved.
7. Removal of gauze is aided by wetting with a few drops of saline, which helps prevent inadvertent removal of the VETSPON® and clot.
8. Remove excess VETSPON® upon achieving hemostasis by gentle irrigation of the site with sterile saline solution to completely wet the sponge.
9. The portion of the VETSPON® that is adhering to the bleeding site may be left in situ. Use only the amount required to achieve hemostasis and remove any excess.
HOW SUPPLIED
For VETSPON® Dental:
Each 1 cm x 1 cm x 1 cm (thickness).
For VETSPON® Flex:
Each 2 cm x 6 cm x 0.7 cm (thickness).
Manufactured by:
Ferrosan Medical Devices A/S. Sydmarken 5, DK-2860 Soeborg, Denmark
Distributed by:
Elanco US Inc., Greenfield, IN 46140, USA
VETSPON® is a registered trademark of Ferrosan Medical Devices A/S.
Elanco and the diagonal bar are trademarks owned or licensed by Eli Lilly and Company, its subsidiaries or affiliates.
4547641
CPN: 2074000.0
Distributed by ELANCO US, INC.
2500 INNOVATION WAY, GREENFIELD, IN, 46140
| Customer Service: | 317-276-1262 | |
| Technical Service: | 800-428-4441 | |
| Website: | www.elanco.us | |
| Email: | elanco@elanco.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
