ZELERIS (Canada)
This treatment applies to the following species: Company: Ceva Animal Health
Company: Ceva Animal Health
florfenicol (400 mg/mL) and meloxicam (5 mg/mL) Injection
Veterinary Use Only
DIN 02501015
Sterile
For subcutaneous use in cattle only.
Structural formula for Florfenicol:
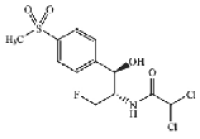
Molecular formula for Florfenicol: C12H14Cl2NO4SF
Molecular mass for Florfenicol: 358.21
Structural formula for Meloxicam:
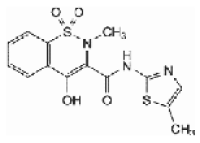
Molecular formula for Meloxicam: C14H13N3O4S2
Molecular mass for Meloxicam: 351.40
Description
ZELERIS® is a clear yellow sterile solution for injection of a synthetic broad-spectrum antibiotic and a non-steroidal anti-inflammatory drug.
Each mL of ZELERIS® contains:
Active ingredients: 400 mg of florfenicol and 5 mg of meloxicam;
Non-medicinal ingredients: 450 mg of dimethyl sulfoxide and glycerol formal (q.s. ad 1 mL).
ZELERIS Indications
For therapeutic treatment of bovine respiratory disease (BRD), with accompanying pyrexia, associated with Mannheimia haemolytica, Pasteurella multocida and Histophilus somni susceptible to florfenicol in beef and non-lactating dairy cattle.
Dosage and Administration
ZELERIS® should be administered as a single subcutaneous dose at a rate of 40 mg florfenicol/kg bodyweight and 0.5 mg meloxicam/kg body weight (i.e. 1 mL/10 kg body weight). Do not administer more than 15 mL at each injection site.
The injection should only be given in the neck area.
To ensure a correct dosage, bodyweight should be determined as accurately as possible to avoid underdosing. For 250 mL and 500 mL vials, the rubber stopper may safely be punctured up to 20 times. Otherwise, the use of a multiple-dose syringe is recommended.
Contraindications
Do not use in animals showing hypersensitivity to florfenicol, meloxicam, or any of the excipients.
Do not use in animals that are dehydrated, or those with renal, cardiovascular, hepatic dysfunction, hemorrhagic or gastrointestinal disorders.
Do not administer concurrently with steroidal, other non-steroidal anti-inflammatory drugs or with anti-coagulant agents.
CAUTIONS:
● Do not exceed the recommended dose.
● The effects of ZELERIS® on bovine reproductive performance, pregnancy and lactation have not been determined.
● Do not use in adult bulls intended for breeding.
● As a class, cyclo-oxygenase inhibitory non-steroidal anti-inflammatory drugs (NSAIDs), such as meloxicam may be associated with gastrointestinal and renal toxicity. Use judiciously when renal impairment or gastric ulceration is suspected. Sensitivity to drug-associated adverse effects varies with the individual animal. Since many NSAIDs may induce gastrointestinal ulceration, concomitant use of ZELERIS® with other anti-inflammatory drugs, such as other NSAIDs and corticosteroids, should be avoided.
● The safety of the product has not been tested in calves under 4 weeks of age.
● Whenever possible, this product should only be used based on antimicrobial susceptibility testing.
Warnings
● Treated cattle must not be slaughtered for use in food for at least 56 days after the latest treatment with this drug.
● Do not use in dairy cattle 20 months of age or older.
● Do not use in calves to be processed for veal. The withdrawal period has not been established in pre-ruminating calves.
● People with known hypersensitivity to florfenicol or meloxicam should avoid contact with this product.
● Dose dependent maternotoxic and foetotoxic effects have been observed in pregnant rats after oral administration of meloxicam. Pregnant women should not handle this product.
● The product is slightly irritant to the eye. Avoid direct contact with skin or eyes. In case of accidental eye exposure, flush with water. In case of accidental skin exposure, wash with soap and water.
● Care should be taken to avoid self-injection. In case of accidental self-injection, seek medical advice immediately and show the package insert to the physician.
● Keep out of reach of children.
Adverse Reactions
Injection site reactions (mostly swelling, induration, heat and pain) were very commonly observed after subcutaneous administration of the product. These effects were transitory and usually resolved without any treatment within 5 to 15 days, but could persist up to 49 days.
Transient inappetence, decreased water consumption, or diarrhea have been reported very rarely following treatment with injectable florfenicol products. Anaphylactic / allergic-type reactions have been reported very rarely with injectable florfenicol products and may require treatment.
Clinical Pharmacology
Florfenicol is a synthetic broad-spectrum antibiotic effective against most Gram-positive and Gram-negative bacteria isolated from domestic animals. Florfenicol acts by inhibiting protein synthesis at the ribosomal level and its action is bacteriostatic and time-dependent. Laboratory tests have shown that florfenicol is active against the most commonly isolated bacterial pathogens involved in bovine respiratory disease which include M. haemolytica, P. multocida and H. somni.
Meloxicam is a non-steroidal anti-inflammatory drug (NSAID) of the oxicam class that acts by inhibition of prostaglandin synthesis, thereby exerting anti-inflammatory, anti-exudative, analgesic and antipyretic effects. It reduces leukocyte infiltration into the inflamed tissue. To a minor extent, it also inhibits collagen-induced thrombocyte aggregation. Meloxicam also has anti-endotoxic properties, because it has been shown to inhibit production of thromboxane B2 induced by endotoxin after administration in calves.
The bioavailability of meloxicam in this combination product is lower compared to the use of meloxicam when administered on its own. The impact of this difference on anti-inflammatory effects has not been investigated in field trials. However, a clear antipyretic effect has been demonstrated in the first 48 hours after administration.
After subcutaneous administration of the product at the recommended dose of 1 mL/10 kg bodyweight, a maximum mean plasma concentration (Cmax) of 4.6 mg/L and 2.0 mg/L occurred 10 and 7 hours after dosing for florfenicol and meloxicam, respectively. Efficacious plasma levels of florfenicol are maintained above the minimum inhibitory concentrations for inhibiting the growth of 90% of organisms (MIC90) of 1.0 μg/mL for M. haemolytica, 0.5 μg/mL for P. multocida and 0.2 μg/mL for H. somni for 72, 120 and 160 hours, respectively. Florfenicol is largely distributed in the whole body and has a low plasma protein binding (approximately 20%). Meloxicam is extensively bound to plasma proteins (97%) and is distributed in all well-perfused organs. Florfenicol is mainly excreted in the urine and to a lesser extent in the faeces with a half-life of about 60 hours. Meloxicam excretion is equally divided between urine and faeces, with a half-life of about 23 hours.
ANIMAL SAFETY:
In a margin of safety study, 32 pre-ruminating calves were enrolled and randomly allocated to treatment groups receiving 0X, 1X, 3X, or 5X of the recommended label dose of ZELERIS® (0.1 mL/kg body weight). Treatments were administered subcutaneously once a week for three consecutive weeks.
In pre-ruminating calves, repeated administration of the recommended dose (1X) was well tolerated, although all the animals in this group experienced transient induration at injection sites, but none exceeded a diameter of 8 cm. As expected, swelling and/or hardness at the injection sites was noted in the animals in the 3X and 5X groups and resolved with time as well.
Repeated weekly administration of overdoses (3X and 5X the recommended dose) in calves was associated with decreased milk consumption, weight loss, loose faeces or diarrhoea. Repeated weekly administration of a 3X dose was fatal in 1 out of 8 calves after the third administration. Repeated weekly administration of a 5X dose was fatal in 7 out of 8 calves after the third administration.
The extent of these adverse effects was dose-dependent. Macroscopic intestinal lesions were observed at necropsy (presence of fibrin, abomasal ulcers, haemorrhagic dots and thickening of the abomasal wall).
An additional margin of safety study was conducted where 32 pre-ruminating calves were randomly allocated to be treated with 0X, 1X, 2X, or 3X of the recommended label dose of ZELERIS®. Treatments were administered as a single subcutaneous injection.
Pain manifested as movement of the head or neck, was noted for all animals treated with ZELERIS® at the time of administration. General health abnormalities (diarrhea and fever) were numerically higher for animals treated with the 2X and 3X doses than animals treated with the 1X dose. Milk consumption and body weights were comparable between all treatment groups. Moreover, there were no differences in hematology parameters, biochemical parameters, urine pH, and urine specific gravity between all treatment groups. Erosions, ulcers and/or discoloration of the mucosa of the stomach were observed in 3, 2, 1, and 4 calves in the 0X, 1X, 2X, and 3X groups, respectively. Therefore, the treatment effect is unclear. Furthermore, there was a dose-related decrease of lymphocytes in the Peyer’s patches of calves in the 2X and 3X treatment groups.
EFFICACY: A total of 329 cattle ranging in age from 2 weeks to 20 months of age were enrolled in a multi-site field trial, including 3 farms in Germany, 1 in Hungary and 1 in Portugal, to assess the efficacy of ZELERIS® in treating signs of BRD associated with M. haemolytica, P. multocida, and H. somni and in reducing associated pyrexia. Animals were enrolled in the trial if they had a rectal temperature ≥40°C and signs of BRD (respiratory distress and depression). Following enrolment, animals were randomly allocated to receive a single subcutaneous injection of either ZELERIS® at the recommended label dose (0.5 mg/kg body weight of meloxicam and 40 mg/kg body weight of florfenicol; 1 mL/10 kg body weight) or florfenicol only (40 mg/kg body weight; 2 mL/15 kg body weight). An animal was considered cured on Day 7 following treatment administration if rescue treatment was not required, rectal temperature was <40°C and animals did not exhibit clinical signs consistent with BRD. Animals with treatment success on Day 7 were considered relapsed if, between Days 8 and 30, they had rectal temperature ≥40°C and signs of BRD (respiratory distress and depression) for 2 consecutive days, required additional treatment for BRD, or died/were euthanized due to BRD.
ZELERIS® was non-inferior to florfenicol as a monotherapy for the treatment of BRD based on the percentage of animals cured on Day 7 (93.9% and 88.5% for ZELERIS® and florfenicol, respectively, P = 0.12). In addition, ZELERIS® was superior to florfenicol alone in reducing rectal temperature at 6 hours post treatment (39.4°C ± 0.41 and 39.9°C ± 0.49, P < 0.0001, respectively). The percentage of animals without pyrexia (rectal temperature < 39.5°C) was higher for animals treated with ZELERIS® than animals treated with florfenicol at 6 hours (53% and 17%, respectively, P < 0.001), on Day 1 (85% and 65%, respectively, P < 0.001), and on Day 2 (86% and 77%, respectively, P = 0.04) post treatment. The percentage of animals that relapsed was similar between animals treated with ZELERIS® and those treated with florfenicol from Days 8 to 14 (9.7% and 8.9%, respectively, P = 0.85) and Days 15 to 30 (16.9% and 15.1%, respectively, P = 0.75).
Storage
Store at room temperature (15°C to 30°C). ZELERIS® has a shelf-life of 28 days after broaching.
How Supplied
ZELERIS® is supplied in translucent multi-layered plastic vials. ZELERIS® is available in vials of 50 mL, 100 mL, 250 mL or 500 mL.
Not all pack sizes may be marketed.
For technical assistance, or to report suspected adverse reactions, call 1-800-510-8864.
Ceva Animal Health Inc., 150 Research Lane, Suite 225, Guelph, ON N1G 4T2
ZELERIS® is a registered trademark of Ceva Santé Animale, France.
December 2022
C746-3120303
A3227-02
CPN: 1221145.1
150 RESEARCH LANE, SUITE 225, GUELPH, ON, N1G 4T2
| Telephone: | 519-650-9570 | |
| Toll-Free: | 800-510-8864 | |
| Fax: | 519-650-9576 | |
| Website: | www.ceva-canada.ca | |
| Email: | service.canada@ceva.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
